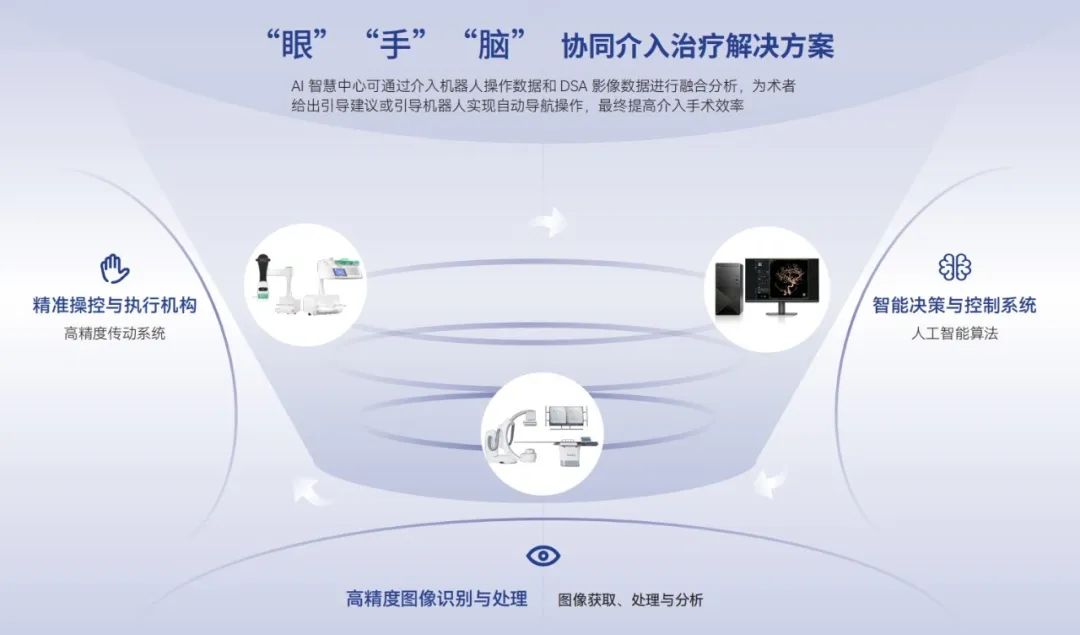
On March 21, 2025, the National Medical Products Administration officially approved the launch of the Weimai Medical ETcath® interventional surgery assistance system - this marks the birth of the world's first interventional surgery robot that realizes the "eye-hand-brain" trinity, and China has completed a historic leap from "following" to "leading" in the field of high-end medical equipment.

With the rapid development of the global medical device market, the clinical requirements and regulatory policies of medical devices in the EU and China, as two major global markets, have attracted much attention. In order to help medical device companies better understand the clinical data requirements of the EU and Chinese markets, AKRA TEAM and LINKS CRO jointly held a webinar focusing on the clinical requirements of medical devices in the EU and China, bringing you the most cutting-edge industry insights and practical guidelines.

For the first time, the online model was adopted to closely connect offices and cities across the country to celebrate this milestone together. The main venue was set up in the Nanjing office, which became a warm harbor for our online gathering.






On April 8, 2024, the "The Venture Capital Day of Shenzhen" and Shenzhen Angel FOF Industry High Quality Development Conference concluded successfully in Futian District, Shenzhen. Co-organized by the Office of the Finance Committee of the CPC Shenzhen Municipal Committee, the Office of the Science and Technology Innovation Committee of the CPC Shenzhen Municipal Committee, and the Shenzhen Bureau of Industry and Information Technology, the event aims to gather global VC resources, explore innovative cooperation models, accelerate the cultivation of new productivity, and promote high-quality development of the industry.

As one of the CROs to be invited to participate in the Tongji Medical Clinical Trial Quality Management Workshop on March 23, 2024, we are pleased to share the highlights of the workshop.