In this issue, a in-depth analysis chapter is specially launched with the intention of analyzing the successful cases of innovative devices underwent clinical evaluation through equivalent comparison and discussing the feasibility of reporting innovative devices through equivalent comparison.
The following case study of clinical evaluation of Patient Monitor (CQZ2000927) is going to be analyzed.
s is well known, clinical evaluation of medical devices can be carried out through multiple pathways, as shown in the above figure. The selection of clinical evaluation pathways for medical devices of different types and risk levels often troubles many regulatory staff or sponsors. Many sponsors have doubts about whether innovative medical devices will be approved for marketing after conducting domestic clinical trials. Here, we hope to provide valuable references for your decision-making.
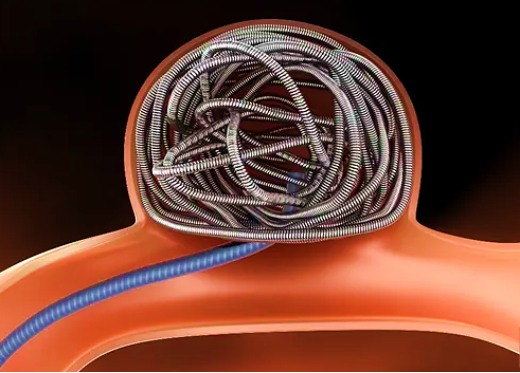
Intracranial aneurysm is a common type of intracranial vascular disease. Due to some factors such as congenital abnormality or acquired injury, the local vascular wall is injured, which is then gradually dilated to form an abnormal bulging under the action of hemodynamic load and other factors. Coil embolization therapy is the most common method of interventional treatment for intracranial aneurysms.In China, the intracranial coil is managed as a medical device of Class III; and clinical evaluation is required.

This year, CCTV 3·15 Evening Party once again exposed the chaos in medical aesthetics industr

Last week, we shared about "follow-up during the epidemic", and learned that follow-up can be carried out in different ways under different circumstances. Secondly, the treatment methods of loss of follow-up and falling off are also explained. So this week, let's talk about SAE during the epidemic.

Ⅰ、 Follow up Today we are going to discuss the subjects who are in the follow-up period